Proteins: We eat it to break it!
Proteins are so important for many different pathways. If we look at it from another perspective we are basically made up of many many proteins, because that is what our genes produce (coded genes to be more specific). Proteins are the translators of our genes, they are the molecules that can be found through out our bodies with various functions.
Okay, first things first proteins are made up of building blocks known as amino acids, there are 20 different amino acids that bind together to produce many different types of proteins via a peptide bond.
A polymer of a protein is referred to as a polypeptide.
Proteins are one of the most complex molecules found in our bodies that serve specific functions. They are highly abundant in our cells with many roles including structural stability, cell communication and signalling, storage, defence and much more.
So, what are proteins?
Proteins are functional molecules that consist of one or more polypeptides that is folded and coiled specifically to a 3-D structure.
Protein structure consists of 4 different levels: primary, secondary, tertiary and quaternary each having unique characteristics and complexity.
From where do get these amino acids? we get them from what we EAT.
I know it's taking the 'you are what you eat' to whole another level but it's true. We get our amino acids from our diets which we then breakdown and bond to make different kinds of proteins.
Before we discuss more about the function and importance of proteins, let's see the amino acid structure.
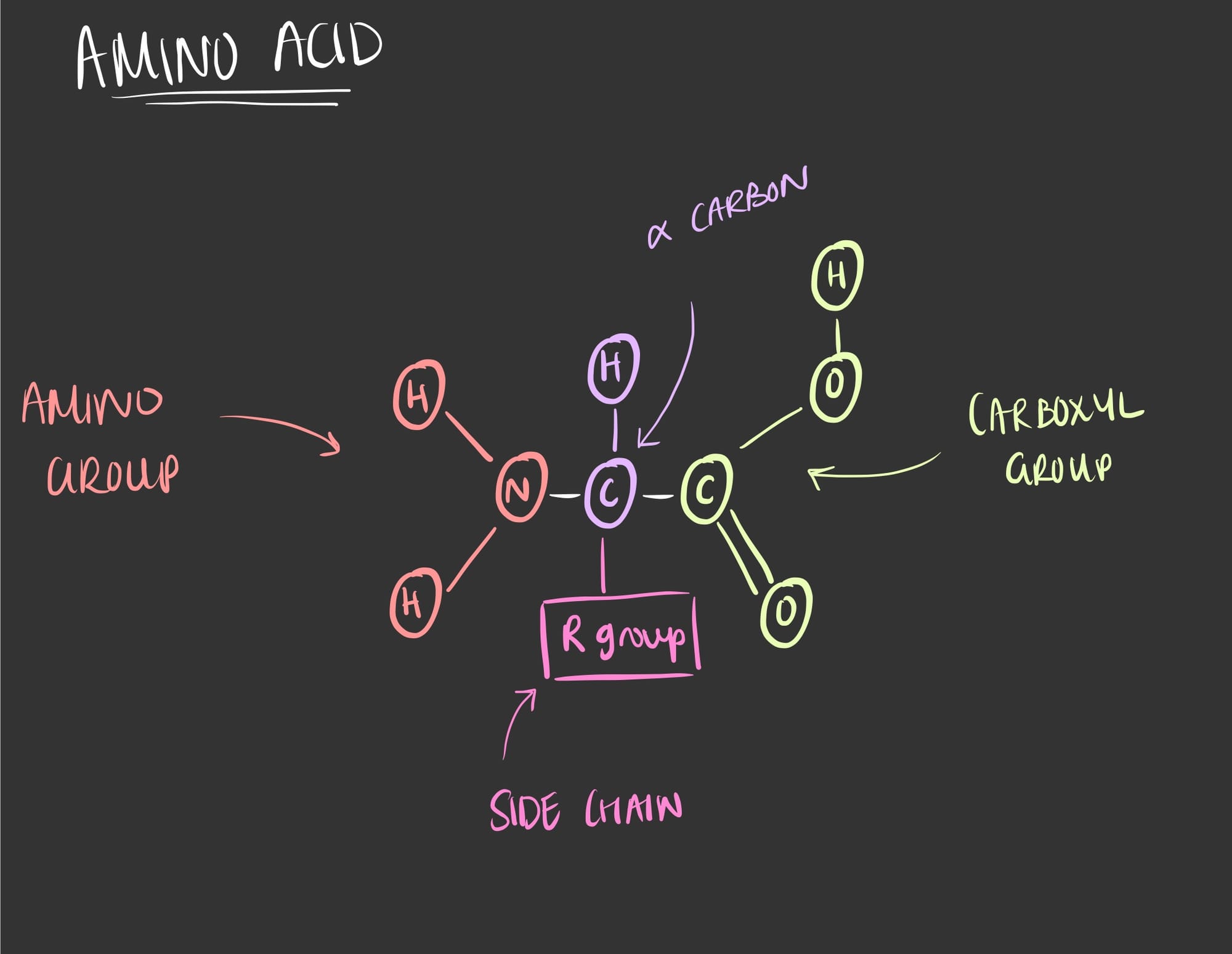
As seen in the figure above, an amino acids consist of an amino group, a carboxyl group, an alpha (⍺) carbon and an 'R' group. The 'R' group is also referred to as the varient group or side chain since it is what makes the 20 different amino acids.
From monomers to polymers - Amino acids edition
Do you remember when we previously discussed about the making and breaking of polymers, that it occurs through two main reactions: dehydration and hydrolysis.
Alright then, now we will look at from the protein perspective.
Polypeptide chain formation is step 1 of the protein structure and function.
So, how is a polypeptide chain formed?
The polypeptide chain is formed via a dehydration reaction. To better understand the concept I made this visual diagram.
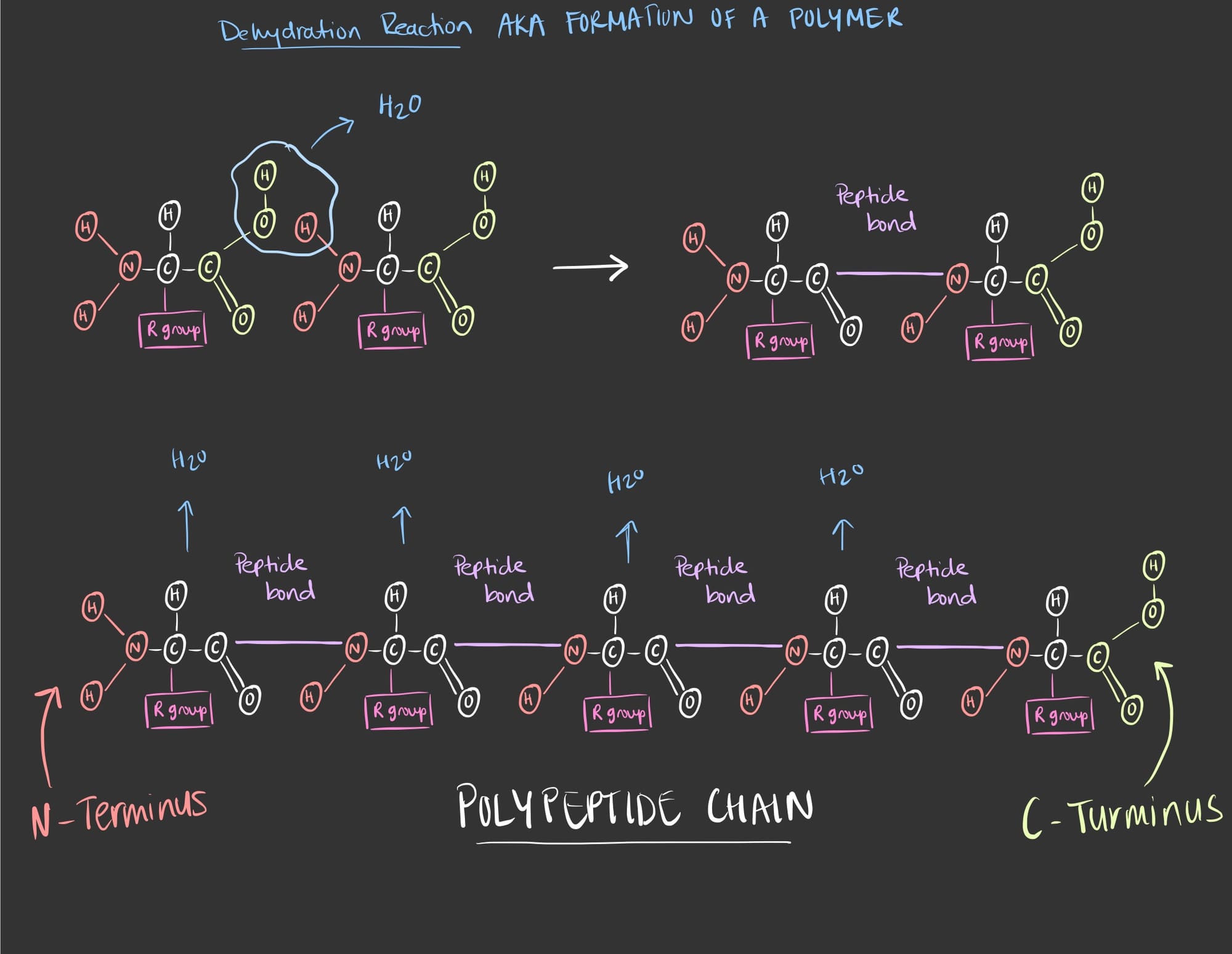
Now, let's put what we see into words.
All amino acids contain three main things, an amine group, a carboxyl group and an alpha (⍺) carbon. When one amino acid binds to another it forms a new peptide bond. The formation of the peptide bond is a result of a dehydration reaction in which a water molecule is released.
Where does the peptide bonds occur?
The peptide bond occurs between the carboxyl group of the first amino acid and the amino group of the second amino acid.
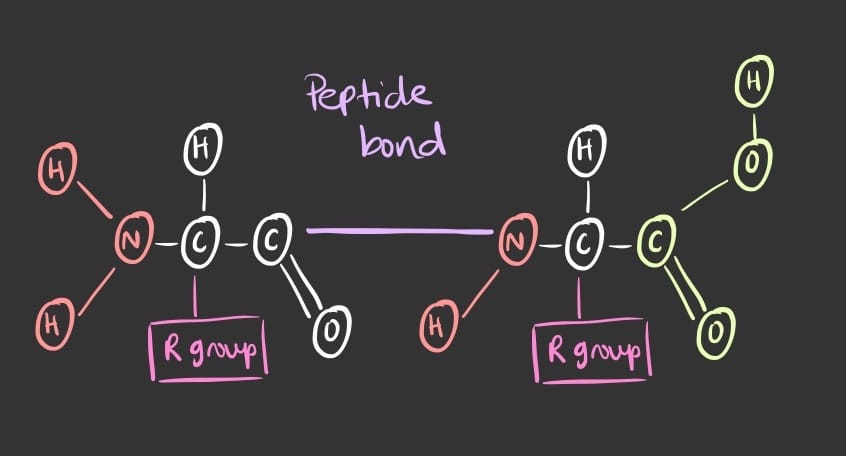
Note: A polypeptide chain will always have a free amino group (N-terminus) on one side and a free carboxyl group (C-terminus) on the opposite side.
More about the 'R' Group
The 20 different amino acids can be differentiated from one other via its 'R' group or the side chain. The 'R' group gives each amino acid a unique property which then contributes to the protein's function and structure.
These 'R' groups can be hydrophilic, hydrophobic, acidic or basic. The peptide chain formation will have what is known as a peptide backbone which consists of the amino group, carboxyl group and alpha (⍺) carbon while the side chain depends on the amino acid added.
Protein structure
Now that we've delved into the basics of amino acids and polypeptide chain formation, let's explore the intricate structure of proteins.
Like a complex puzzle, proteins fold and coil into specific 3-D structure, crucial for their diverse functions.
This might be the most complicated part of the proteins segment but it's nothing that can't be broken down to simple words.
- Primary Structure: The primary structure of a protein refers to the linear sequence of amino acids in a polypeptide chain. This sequence is determined by the genetic code encoded in DNA (more on this in gene transcription & translation). Even a slight alteration in the sequence can lead to significant changes in the protein structure and function.
- Secondary Structure: As the polypeptide chain begins to fold, secondary structures such as alpha (⍺) helices and beta (β) sheets emerge. These structures are stabilized by hydrogen bonds between amino acids, forming recurring patterns along the chain.
- Tertiary Structure: The tertiary structure represents the overall three-dimensional (3D) shape of a single polypeptide chain. The structure is determined by interactions between the amino acids side chains aka the 'R' groups, including hydrogen bonds, di-sulfide bonds, hydrophobic interactions, and electrostatic attractions.
- Quaternary Structure: Some proteins consist of multiple polypeptide chains that come together to form a functional protein complex. The arrangement and interaction of these subunits define the quaternary structure, which is essential for the protein's biological activity. An example of the quaternary structure is the protein Hemoglobin, an oxygen binding protein of the red blood cells.
Let's check out this simplified sketch of the 4 levels of protein structure.
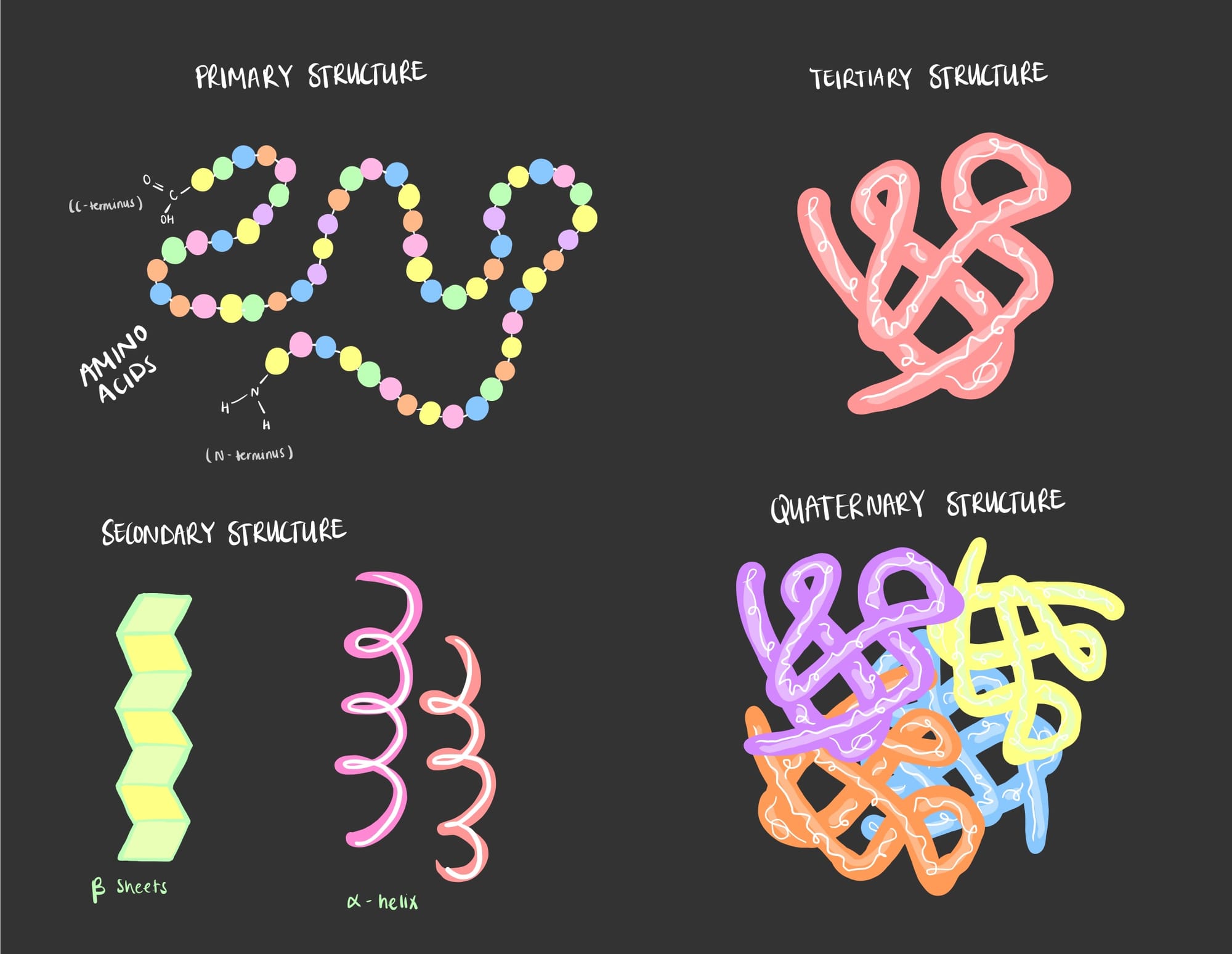
Note: a protein can be functional in the tertiary structure and can proceed into its designated functional location. Not all proteins are quaternary in structure, only the complex ones.
In summary, protein structure is hierarchical, with each level contributing to the overall shape and function of the molecule. The precise folding and arrangement of amino acids are essential for proteins to carry out their diverse roles in cellular processes, from enzyme catalysis to structural support and more.