Lipids: The misunderstood enemy
Lipids are one of the most energy providing macromolecule. While many remain afraid of the idea of lipids and fats it is crucial to note it's significance for many biological processes.
Here is a shocking fact, lipids constitute about 50% of a cell’s mass, yes you read that right 50%. It is found in many forms in the cell (more about that later).
Lipids come in many forms such as fatty acids, triglyceride, phospholipids, steroids and more. Lipids give us energy due to the breakage of the hydro-carbon bonds.
Well to understand let’s look at the structure and formation of the simplest form aka the monomer of lipids which is triglyceride.
Triglyceride - simplest form of lipids
Triglyceride is the most abundant lipid in your body and it’s the most consumed type of lipids. It consists of a glycerol molecule and 3 fatty acids, the glycerol acts as the backbone.
The glycerol and fatty acids are the monomers of triglycerides.
Fatty acids
To understand let us look at the structure of a fatty acid, a main component in the triglyceride molecule.
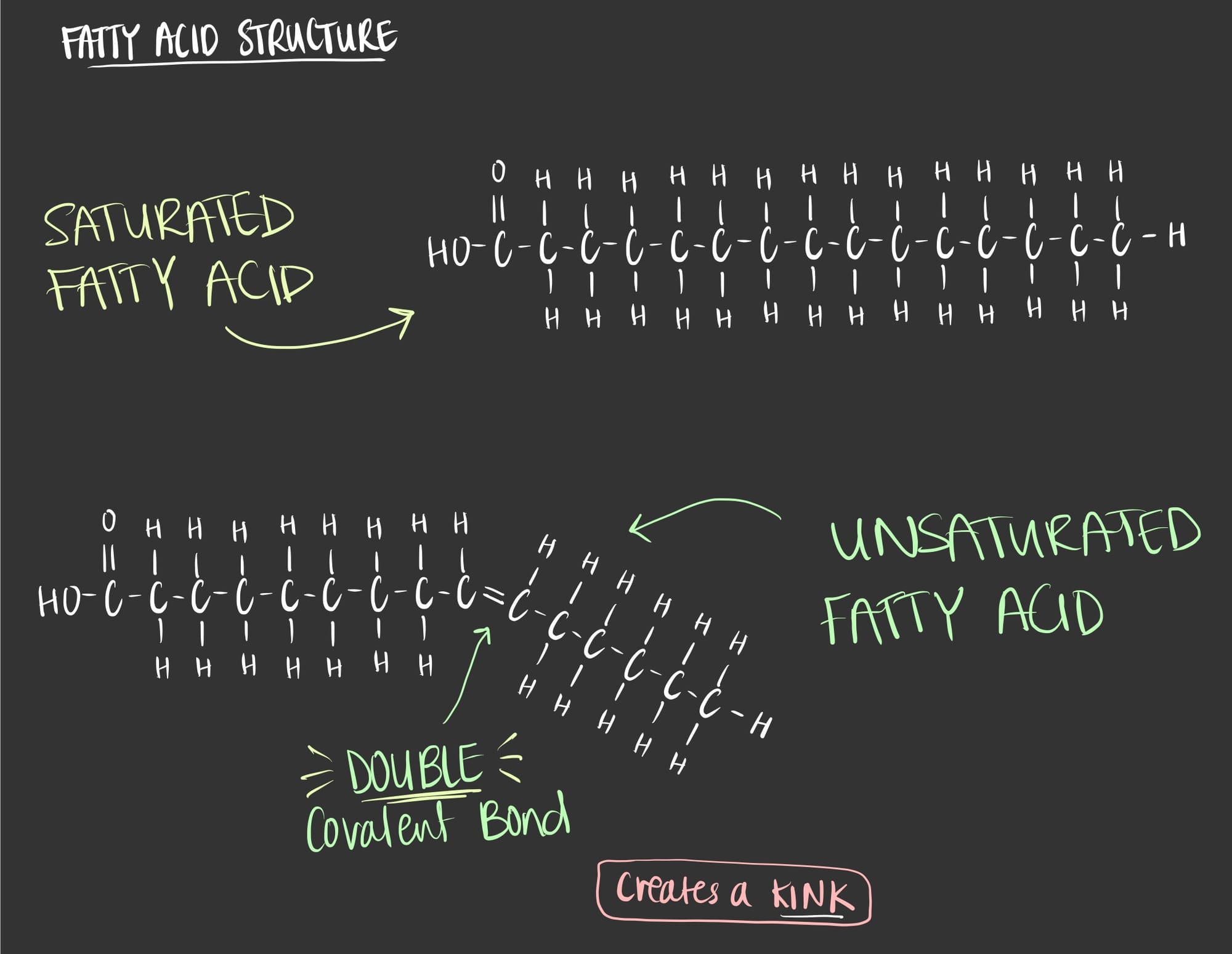
A fatty acid is a chain of hydrocarbons, in other words it’s a carbon that is bonded to a hydrogen which is bonded to a carbon that is bonded to a hydrogen and so on. It is the simplest form of lipids and it is utilized to synthesize other molecules such as triglycerides and lipids.
As seen in the figure above, a fatty acid molecule can be saturated and unsaturated. The saturation here refers to the bonding of carbons to hydrogens.
SATURATED fatty acids have only single covalent bonds between the carbon atoms which gives them a linear structure. The linear structure allows the fatty acid molecules to pack closely which is seen in butter. At room temperature butter can remain intact (aka solid) due to the chemical structure of the saturated fatty acids.
UNSATURATED fatty acids have double covalent bonds between the carbon atoms which gives them a kink. The kink doesn’t allow the fatty acid molecules to pack closely which is seen in oil. At room temperature oil is liquid due to the chemical structure of the unsaturated fatty acids.
Glycerol
A glycerol is an alcohol that consists of three carbons, eight hydrogens and three oxygens. This molecule (glycerol) plays as the backbone of many complex molecules such as triglyceride and phospholipids.
To visualize the glycerol molecule check out the figure below.
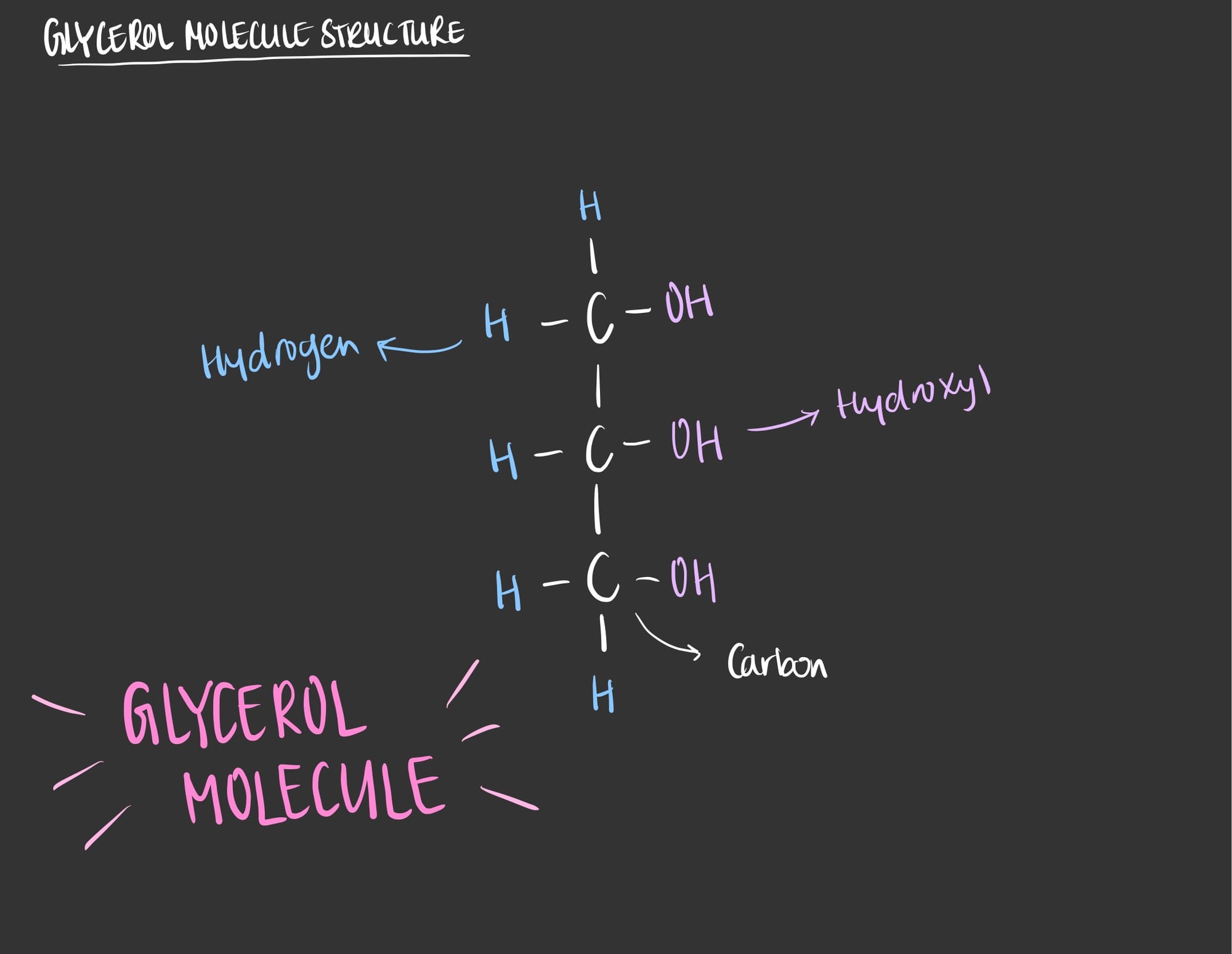
Triglyceride synthesis
Well now let’s see how these two molecules (glycerol and fatty acids) bind to form one triglyceride molecule. The glycerol molecule acts as a backbone for the fatty acids, it has 3 central carbons which allows for 3 fatty acids to attach to it.
The synthesis of a triglyceride occurs through a chemical reaction known as a Dehydration Synthesis Reaction. To simply understand the reaction let’s look at the following illustration.
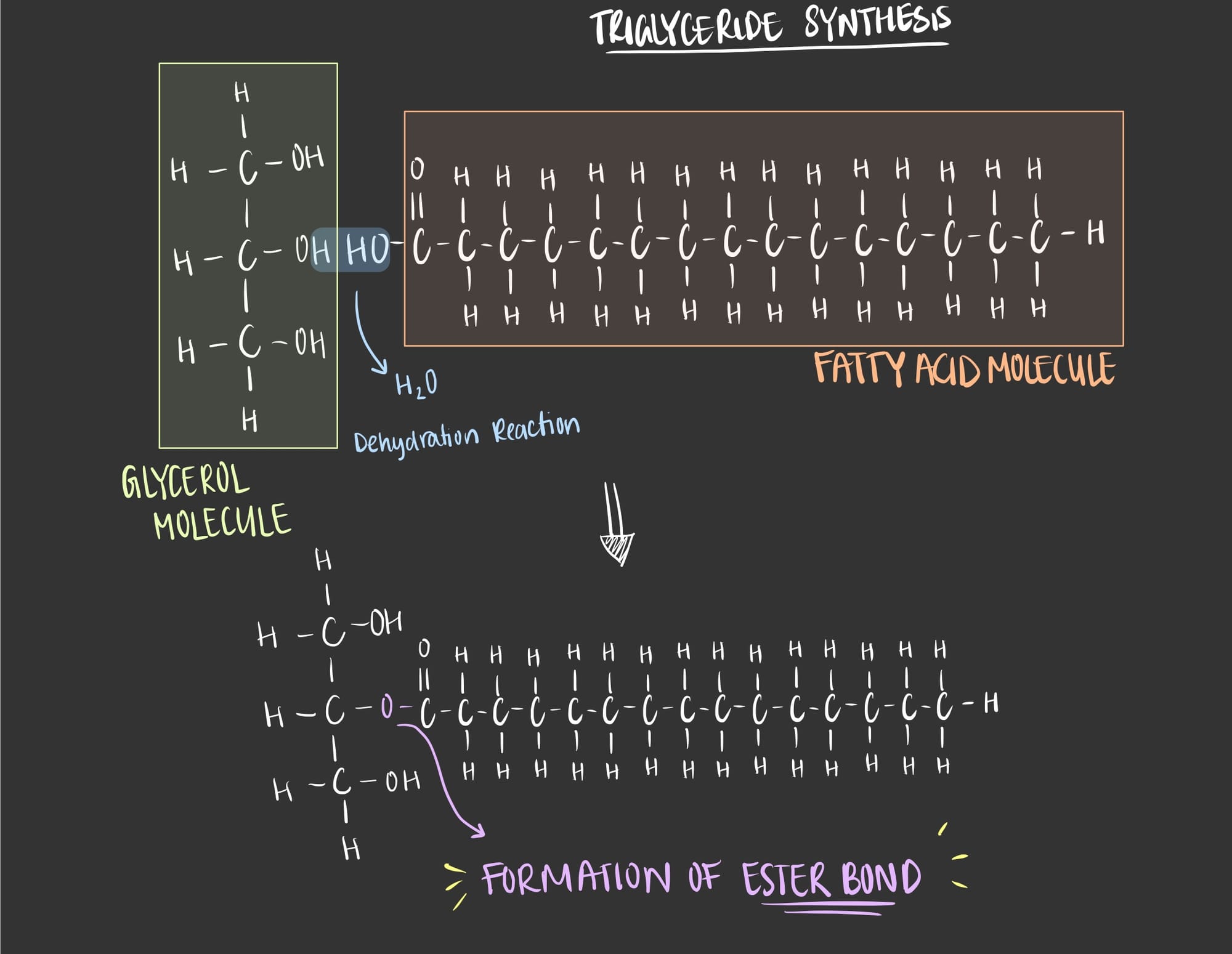
As seen in the image, the synthesis of a triglyceride is through a very simple chemical reaction known as a dehydration synthesis reaction. In this reaction, water is lost in order to form a bond between the glycerol molecule and the fatty acid. The formed bond is called an ester bond.
So, this is the final structure of a triglyceride.
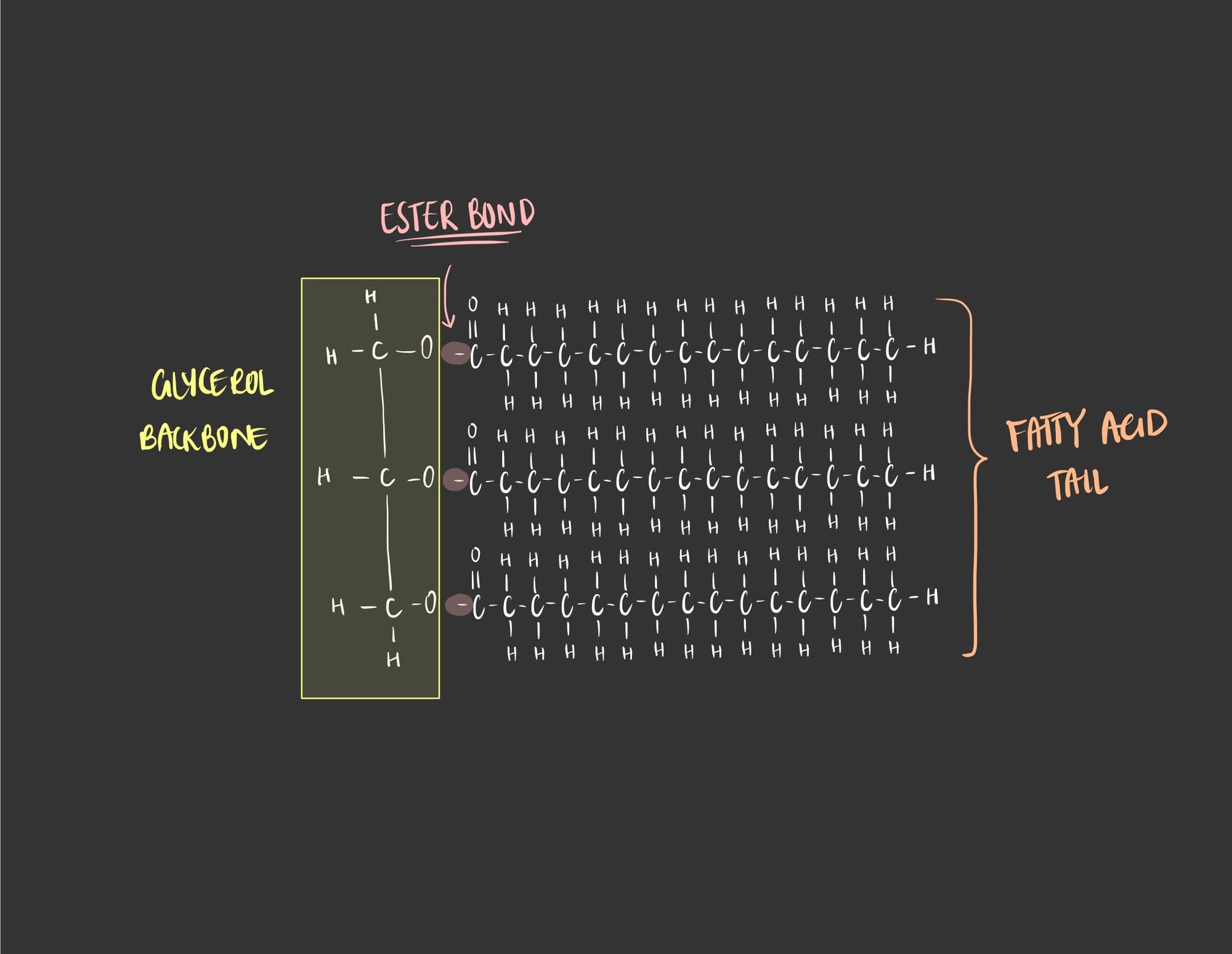
This chemical reaction is reversible, in which water is added breaking the ester bond and therefore separating the two molecules. This process is called hydrolysis, where a water molecule is added between the two molecules preventing it from forming a bond between them. So, a triglyceride molecule will be broken into a glycerol molecule and a fatty acid molecule.
Phospholipids - mostly found in the cell membrane
Similar to triglyceride, phospholipids have a glycerol molecule as its backbone and 2 fatty acids attached to 2 carbons. The difference lies in the bonding to the carbon, where 2 carbons are attached to 2 fatty acids, and the third carbon is attached to a phosphate group (PO4-3).
The phosphate group gives the phospholipid its negative charge.
Now let us look at the structure of the phospholipid.
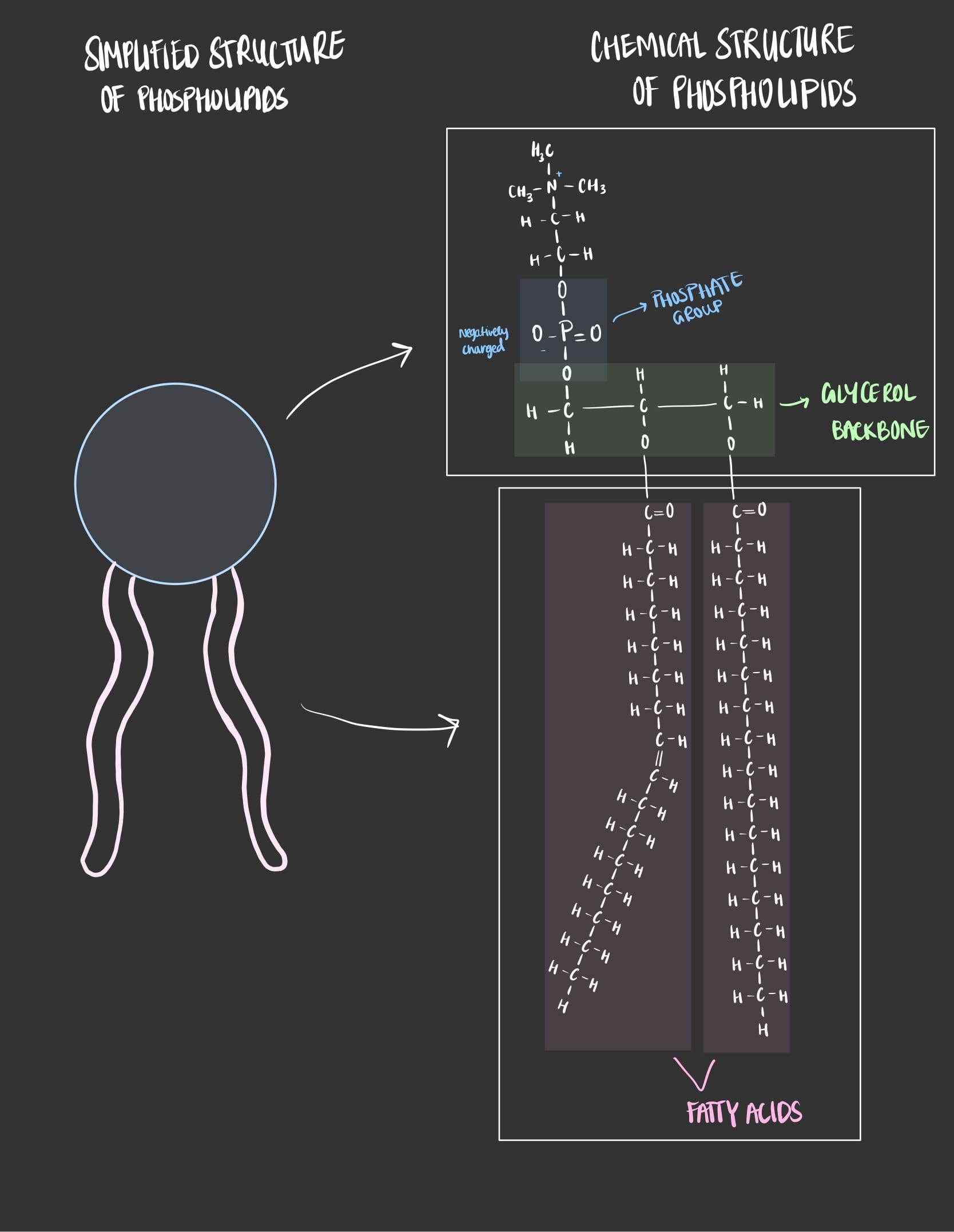
As seen the figure above, phospholipids are made of a ‘head’ which contains a phosphate that gives the head part a negative charge. The head part is also water loving which means that it has no problem interacting with water, when a molecules is water loving then it is called hydrophilic.
While the ‘tail’ consists of two fatty acids which doesn’t contain any charge. The tail part is water hating which means that it doesn’t like to interact with water, when a molecule is water hating then it is called hydrophobic.
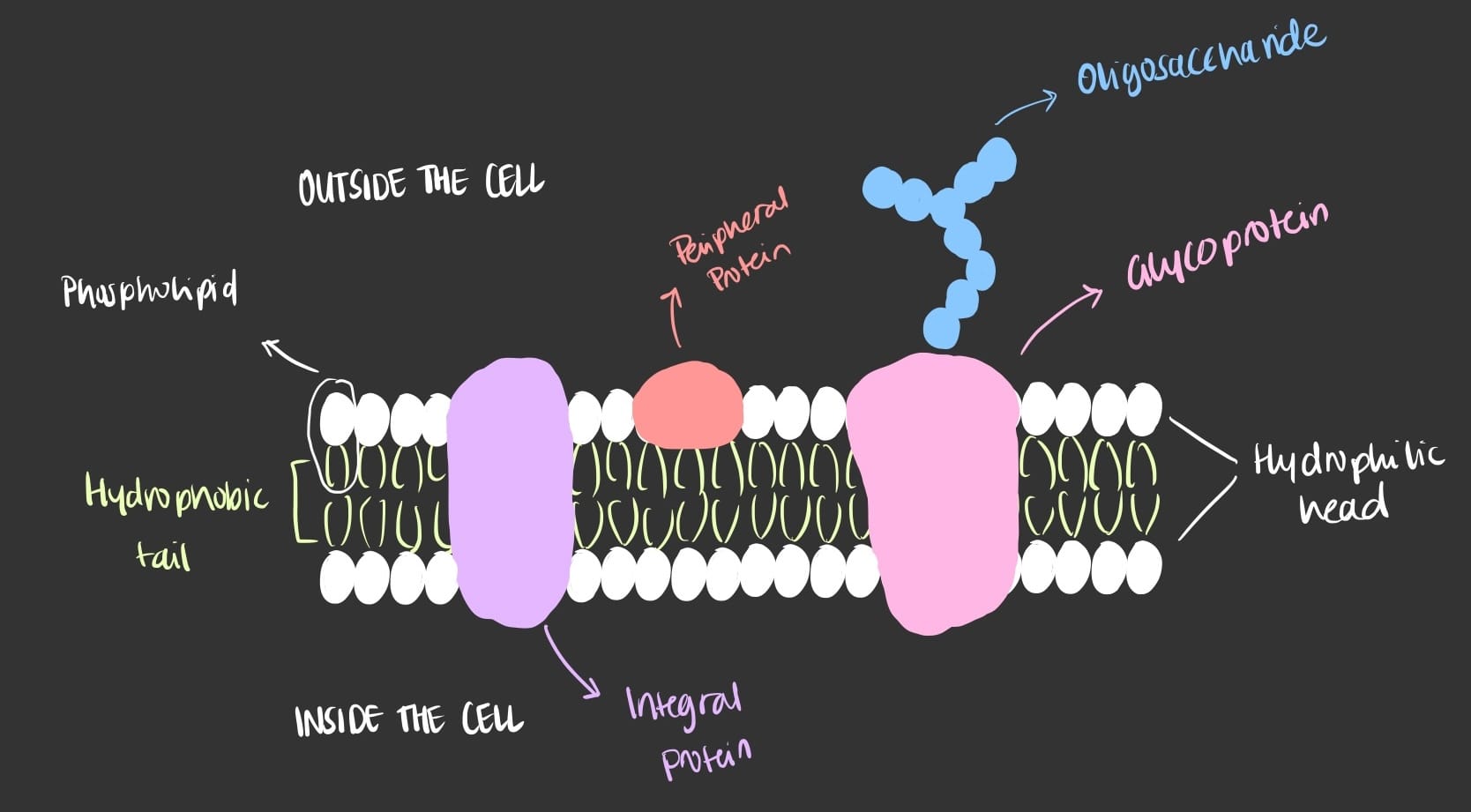
Phospholipids are found abundantly in the cell membrane in which they are arranged in a specific way due to the hydrophobic and hydrophilic nature of the head and tail. The head of the phospholipid will always face the outer and inner of the cell since it can interact with water while the tails will always face each-other since it can’t interact with water. This arrangement of the cell membrane is known as the phospholipid bilayer.
The cell membrane plays a pivotal role in not only maintaining cell structure and shape but also controls what enters and leaves the cell. The cell membrane has many molecules attached to it’s inner and outer membranes such as proteins and lipids that have crucial functions. Thus, making the cell membrane semipermeable, highly selective of what enters and exists the cell.
Here is a simplified illustration of the cell membrane with the different molecules attached to it.
Steroids - the natural ones
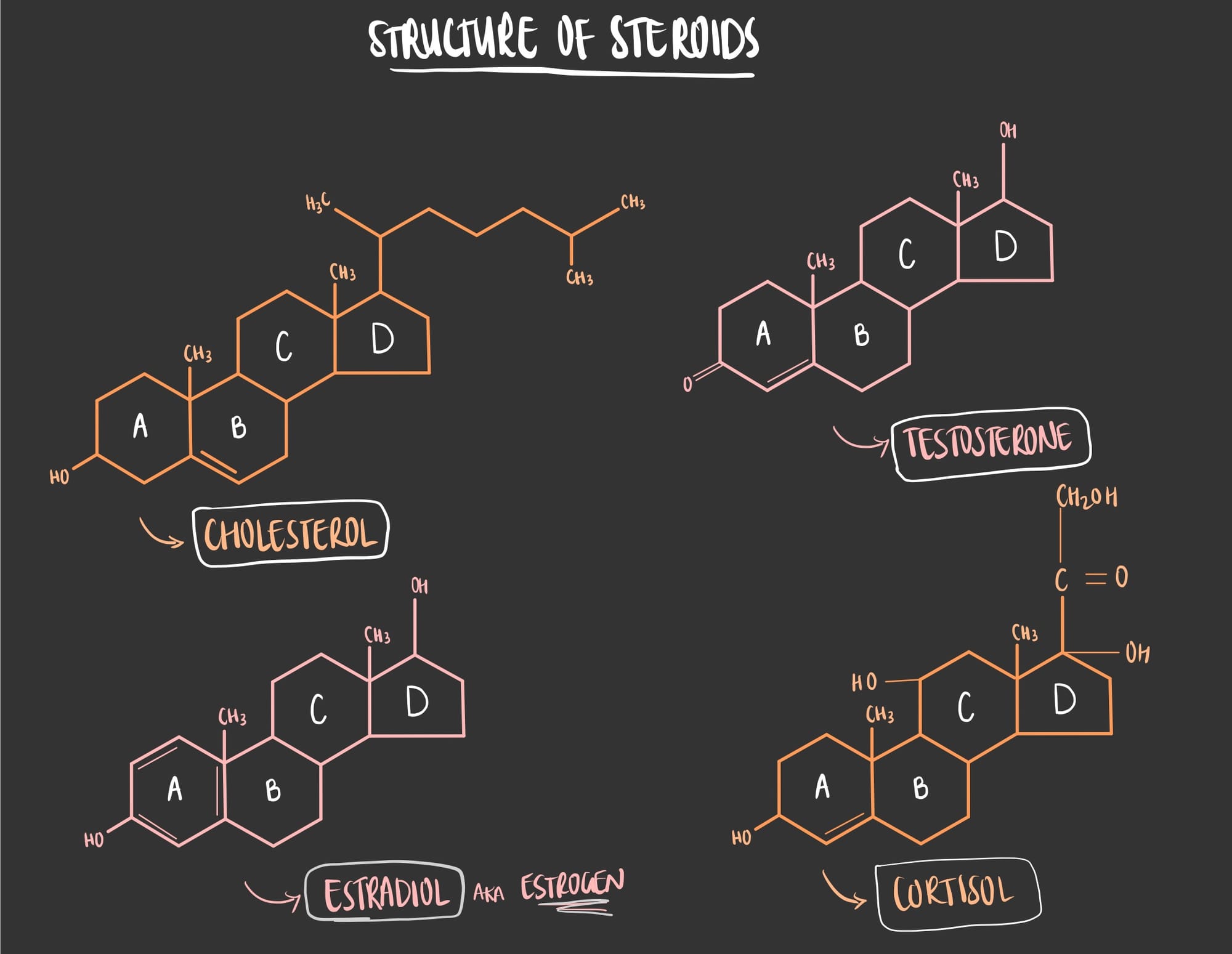
Unlike triglycerides, steroids have a different structure in which it consists of 4 rings of carbon atoms (in chemistry terms it’s called a cycloalkane rings).
Cholesterol
One of the most important and common steroids is Cholesterol. It is one of the most crucial molecules that the human body produces in the liver and should be consumed in your diet. Yes, against all the bad press that cholesterol receives, it is actually very important for many biological process. Cholesterol highly affects the fluidity of the cell in which its presence in the cell membrane prevents the cell membrane from being too rigid in cold conditions and too fluid in warm conditions.
Another crucial function of cholesterol is that it is utilized in the synthesis of many other lipid molecules. For example cholesterol is the precursor of vitamin D.
Other examples of steroids:
- Cortisol: helps in maintaining blood sugar.
- Bile salts: crucial in lipid digestion and absorption.
- Vitamin D: important for bone growth.
- Estrogen & Progesterone: important for sexual function.
Other types of lipids
There are also other types of lipids that play important roles in many biological functions. Some of these lipids are:
- Prostaglandins - these lipids have a variety of functions which includes: prevent stomach ulcers, regulate body temperature, modify hormones that contribute to the inflammatory response, dilate airways to the lungs and influence formation of blood clots.
- Fat-soluble vitamins - Beta-carotene which help in converting yellow-orange pigments (that can be found in egg yolks and tomatoes) in to vitamin A. This category also includes vitamin D, E, & K. Lastly, it also includes lipoprotein which are HDL, LDL and VLDL that help in transporting lipids in the bloodstream.